Longevity: Exploring the Science of a Longer, Healthier Life
Chapter III: Sensitive Topic That Needs To Be Discussed: Weight
Weight has become quite a taboo topic in clinical medicine these days. I’ll admit that during my time in traditional primary care, I often requested and reviewed patients’ vital signs—including height and weight—but rarely addressed their weight directly unless it was extremely high or low. This avoidance was, in part, because I lean non-confrontational and because diagnosing someone with obesity often felt like labeling them unfairly or passing judgment. Adding to this discomfort was the reality that time constraints often reduced any meaningful conversation to a quick, generic statement like, “You need to work on your lifestyle with diet and exercise,” followed by an implicit tone of doom: “Or else—shame, failure, and more doom!”
To complicate matters further, even if patients expressed interest in addressing their weight, I would have to inform them that “the blockbuster weight-loss medication that seems to be helping everyone isn’t covered by your insurance.” It’s no wonder some patients outright refuse to have their weight recorded. When my medical assistant informed me that patient A or B had declined to be weighed, I often replied with a relieved “Great!”—not because I didn’t care, but because it gave me an excuse to avoid what often felt like an awkward and uncomfortable discussion.
This reluctance is particularly concerning in light of the data. According to the National Center for Health Statistics, data from 2017 to 2020 indicate that 73.6% of American adults aged 20 and older were overweight or obese, with 41.9% meeting the criteria for obesity (Centers for Disease Control and Prevention [CDC], 2022). These statistics underscore the importance of addressing weight in a sensitive, effective, and patient-centered manner, as it impacts such a large portion of the population.
Maintaining a healthy weight is essential for achieving and preserving overall well-being. Body weight significantly impacts the functioning of almost every system in the body, influencing the risk of chronic diseases, the ability to perform daily activities, and overall quality of life. Understanding what constitutes a healthy weight, the risks of deviation from it—whether in the form of obesity or undernutrition—and the steps necessary to achieve it can empower individuals to make informed health decisions. This chapter explores the tools used to define weight categories, the medical evidence linking weight to health outcomes, and practical strategies for achieving and sustaining a healthy weight.
Defining Weight Categories: Tools and Metrics
One of the most commonly used tools to define weight categories is the Body Mass Index (BMI). BMI is a simple calculation based on an individual's weight in kilograms divided by the square of their height in meters (kg/m²). This widely adopted metric categorizes individuals as underweight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese (BMI ≥30.0), or morbidly obese (BMI ≥40.0) (Centers for Disease Control and Prevention [CDC], 2020). While BMI provides a convenient and quick way to assess general weight status across populations, it has significant limitations. It does not account for variations in muscle mass, fat distribution, or bone density (Heymsfield & Wadden, 2017).
To address the shortcomings of BMI, more advanced methods have emerged for assessing body composition. A Dual-Energy X-Ray Absorptiometry (DEXA) scan is considered one of the most accurate tools for measuring fat mass, lean mass, and bone density. Unlike BMI, DEXA provides detailed insight into an individual’s fat distribution, which is a critical factor in evaluating health risks (Shuster et al., 2012). Other tools, such as skinfold calipers and bioelectrical impedance analysis (BIA), offer practical alternatives for estimating body fat percentage, although they are less precise (Heymsfield & Wadden, 2017). Waist circumference and the waist-to-hip ratio are additional measures that highlight the risks associated with central obesity, which is closely linked to metabolic syndrome and cardiovascular diseases (Ashwell & Gibson, 2016).
Emerging techniques, including 3D body scanning and imaging technologies, hold promise for even greater accuracy in assessing body composition. These methods are increasingly being explored in clinical and research settings as tools to provide a more nuanced understanding of body weight and health (Shuster et al., 2012).
Obesity and Its Associated Health Risks
Obesity is a multifaceted condition linked to a wide array of chronic diseases. Understanding the specific mechanisms and statistical relationships between obesity and these conditions allows for more informed prevention and management strategies. Below, we explore the associations between obesity and several key health issues, supported by data on risk ratios (RR) and odds ratios (OR) from peer-reviewed research.
Before diving in, it’s helpful to understand what these statistical tools mean:
Risk Ratio (RR): This measures how much more likely an outcome is to occur in one group compared to another. For example, if the risk ratio for developing diabetes in people with obesity is 4.6, it means they are 4.6 times more likely to develop diabetes than those without obesity (Grant, 2014).
Odds Ratio (OR): This compares the odds of an outcome happening in one group versus another. While similar to risk ratio, odds ratio is often used in studies with smaller sample sizes or rare events. If the odds ratio for a condition is 2.0, it means the odds of that condition are twice as high in the exposed group compared to the unexposed group (Norton, Dowd, & Maciejewski, 2018).
These tools help clinicians and researchers quantify the strength of the relationship between obesity and various health conditions, offering valuable insights for clinical and public health strategies.
1. Obesity and Heart Disease
One of the most well-documented consequences of obesity is its impact on cardiovascular health. Studies consistently demonstrate a strong association between obesity and hypertension, atherosclerosis, and myocardial infarction (Lavie et al., 2009). Central obesity, characterized by an accumulation of visceral fat around internal organs, is a key component of metabolic syndrome, which increases the risk of both cardiovascular disease and type 2 diabetes (Ashwell & Gibson, 2016).
Excess body fat contributes to high blood pressure, elevated cholesterol levels, and systemic inflammation, all of which damage the heart and blood vessels. At the molecular level, visceral fat releases inflammatory cytokines that promote atherosclerosis (plaque buildup in arteries). This condition narrows blood vessels, increasing the likelihood of a heart attack or stroke.
Risk Ratios: Obesity is associated with a 2.1-fold increased risk (RR: 2.1) of developing coronary artery disease compared to normal-weight individuals (Lavie et al., 2009).
Other Risks: Obesity increases the odds of heart failure by 1.96 times (OR: 1.96) (Kenchaiah et al., 2002).
2. Obesity and Diabetes
Type 2 diabetes is one of the most significant health consequences of obesity. Excessive fat accumulation disrupts the body’s ability to use insulin effectively, leading to elevated blood sugar levels and, ultimately, insulin resistance (CDC, 2020).
Insulin is a hormone produced by the beta cells in the pancreas, released in response to rising blood sugar levels. It functions like an efficient password manager, unlocking millions of cells throughout the body. Once these cells are "unlocked," they absorb sugar (glucose) from the blood and use it as an energy source. This process helps maintain blood sugar levels within a healthy range.
In insulin resistance, however, this "password manager" becomes much less effective. Instead of opening millions of cells to take up glucose, it may only unlock hundreds of thousands—or worse, just tens of thousands or even less. To compensate, the pancreas produces more insulin to force cells to respond. Over time, this overproduction places significant stress on the pancreas, causing it to become less efficient. Eventually, the pancreas can no longer keep up with the demand for insulin, leading to its failure. At this stage, the individual becomes fully dependent on external insulin sources for survival.
Obesity-induced insulin resistance is driven by multiple mechanisms, including the release of free fatty acids and inflammatory molecules from excess fat tissue. These substances interfere with insulin signaling pathways, further compounding the problem. Addressing obesity is therefore crucial to preventing or managing type 2 diabetes and its complications.
Risk Ratios: The risk of developing type 2 diabetes increases dramatically with obesity:
For those with a BMI ≥30, the risk is 4.6 times higher (RR: 4.6) compared to individuals with a BMI <25 (Hu et al., 2001).
For morbidly obese individuals (BMI ≥40), the risk is as high as 7.6-fold (RR: 7.6) (CDC, 2020).
3. Obesity and Cancer
Obesity is strongly linked to the development of several types of cancer, with mounting evidence from epidemiological and clinical studies. Among the cancers most associated with obesity are endometrial, breast (postmenopausal), colorectal, esophageal adenocarcinoma, pancreatic, kidney, liver, ovarian, and gallbladder cancers. While the exact mechanisms underlying these associations are not fully understood in all cases, key contributors include chronic low-grade inflammation, hormonal imbalances, metabolic dysfunction, and alterations in growth factor signaling pathways. In the next paragraphs, I’ll discuss current understanding on how obesity may contribute to cancers, highlight some of the obesity associated cancers, and summarize the data in risk.
Mechanisms of Obesity-Associated Cancer
Fat tissue is biologically active, producing hormones (like estrogen) and inflammatory molecules (such as cytokines) that create an environment conducive to cancer initiation and progression:
Hormonal Effects: Adipose tissue produces estrogen through the conversion of androgens by the enzyme aromatase. Elevated estrogen levels are known to stimulate the growth of hormone-sensitive cancers, such as breast and endometrial cancers (Lauby-Secretan et al., 2016).
Inflammation: Obesity is characterized by chronic low-grade inflammation, mediated by inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). This inflammatory environment may contribute to DNA damage, promote tumor cell proliferation, and inhibit apoptosis (Bhaskaran et al., 2014).
Insulin and Growth Factors: Obesity is associated with hyperinsulinemia and increased levels of insulin-like growth factor-1 (IGF-1), both of which promote cell division and inhibit cell death, processes that can lead to cancer (Renehan et al., 2008).
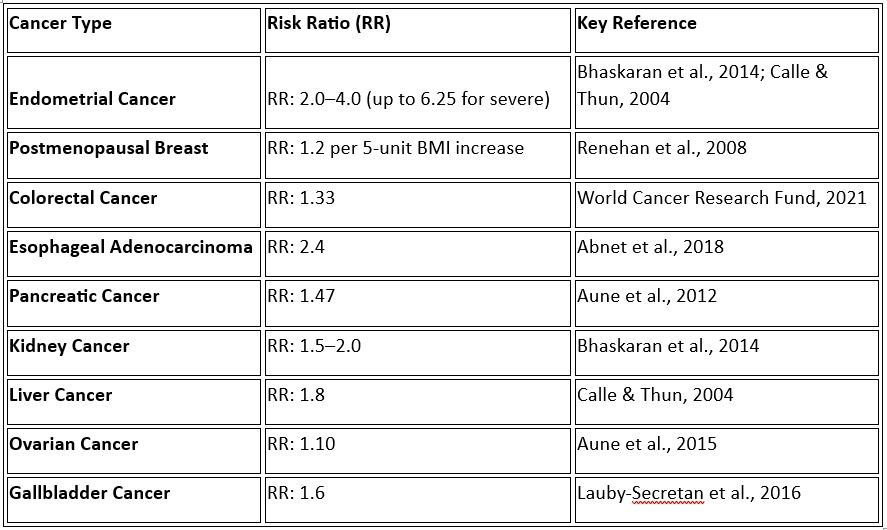
Risk Ratios for Obesity-Associated Cancers
a. Endometrial Cancer
Obesity is one of the strongest risk factors for endometrial cancer, particularly in postmenopausal women. Excess adipose tissue elevates estrogen levels, which can stimulate the endometrial lining and promote malignant changes.
Risk Ratios: Women with obesity have a 2 to 4 times increased risk (RR: 2.0–4.0) compared to those of normal weight (Bhaskaran et al., 2014). Severe obesity (BMI ≥40) is associated with an even higher risk, with a 6.25-fold increase (RR: 6.25) (Calle & Thun, 2004).
b. Breast Cancer (Postmenopausal)
Obesity is linked to an increased risk of breast cancer in postmenopausal women, likely due to elevated estrogen and inflammatory markers. However, premenopausal obesity may have a protective effect, possibly due to differences in hormonal profiles.
Risk Ratios: For postmenopausal women, the risk of breast cancer increases by 20% (RR: 1.2) for every 5-unit increase in BMI (Renehan et al., 2008). Additionally, women with severe obesity (BMI ≥40) face a 58% increased risk (RR: 1.58) compared to women of normal weight (Neuhouser et al., 2015).
c. Colorectal Cancer
Colorectal cancer is associated with obesity in both men and women, with visceral fat and insulin resistance believed to play key roles.
Risk Ratios: Obesity increases the risk of colorectal cancer by 33% (RR: 1.33) in both sexes (World Cancer Research Fund, 2021). For every 5-unit increase in BMI, the risk rises by 18% (RR: 1.18) (Renehan et al., 2008).
d. Esophageal Adenocarcinoma
Obesity is a major risk factor for esophageal adenocarcinoma, partly due to increased rates of gastroesophageal reflux disease (GERD) and Barrett’s esophagus in obese individuals.
Risk Ratios: Obesity increases the risk of esophageal adenocarcinoma by 2.4-fold (RR: 2.4) (Abnet et al., 2018).
e. Pancreatic Cancer
The association between obesity and pancreatic cancer may be mediated by insulin resistance and chronic inflammation, but the exact mechanisms are less well understood.
Risk Ratios: Obesity increases the risk of pancreatic cancer by 47% (RR: 1.47) (Aune et al., 2012).
f. Kidney Cancer
Excess fat increases the risk of renal cell carcinoma, likely through hormonal and inflammatory pathways.
Risk Ratios: Obesity is associated with a 1.5- to 2.0-fold increased risk (RR: 1.5–2.0) for kidney cancer (Bhaskaran et al., 2014).
g. Liver Cancer
Obesity contributes to liver cancer risk through mechanisms such as fatty liver disease and chronic inflammation.
Risk Ratios: The risk of hepatocellular carcinoma is 1.8 times higher (RR: 1.8) in obese individuals (Calle & Thun, 2004).
h. Ovarian Cancer
The relationship between obesity and ovarian cancer is more complex and less consistent, but recent studies suggest an increased risk in postmenopausal women.
Risk Ratios: Obesity is associated with a 10% increased risk (RR: 1.10) (Aune et al., 2015).
i. Gallbladder Cancer
Obesity increases the risk of gallbladder cancer, likely due to its role in gallstone formation and chronic inflammation.
Risk Ratios: The risk of gallbladder cancer is 1.6 times higher (RR: 1.6) in individuals with obesity (Lauby-Secretan et al., 2016).
Summary Table 1: Risk Ratios for Obesity-Associated Cancers
Description: The association between obesity and various cancers reported through risk ratio (RR) underscores the importance of weight management as part of cancer prevention strategies. While mechanisms such as inflammation, hormonal imbalance, and metabolic dysfunction are implicated, further research is needed to clarify specific pathways for certain cancers. Nonetheless, the evidence supports obesity as a significant modifiable risk factor for cancer, making its prevention and management critical in reducing the global cancer burden.
4. Obesity and Non-Alcoholic Fatty Liver Disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent and increasingly recognized condition that encompasses a spectrum of liver abnormalities. It is characterized by excessive fat accumulation (steatosis) in the liver, occurring in individuals who consume little to no alcohol. NAFLD ranges from simple steatosis (benign fat deposition) to more severe conditions such as non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC). Obesity is the most significant risk factor for NAFLD, as it promotes the deposition of fat in the liver and exacerbates metabolic dysregulation.
Definition and Diagnostic Criteria
NAFLD is defined as hepatic steatosis (≥5% of liver cells containing fat) that occurs without significant alcohol consumption (typically defined as <20 g/day for women and <30 g/day for men), other known causes of liver disease, or long-term use of steatogenic medications (Chalasani et al., 2018). The disease can be divided into two primary categories:
Simple Steatosis: Fat accumulation without inflammation or fibrosis. This condition is relatively benign but may progress to more severe forms.
Non-Alcoholic Steatohepatitis (NASH): Steatosis accompanied by liver inflammation, cellular injury, and varying degrees of fibrosis. NASH is a progressive condition that may lead to cirrhosis or liver cancer.
Epidemiology and Future Projections
NAFLD is currently the most common cause of chronic liver disease globally, with prevalence rates rising in parallel with obesity and metabolic syndrome.
Global Prevalence: An estimated 25% of the world's population has NAFLD, with higher rates in regions with high obesity prevalence, such as North America (24%) and the Middle East (32%) (Younossi et al., 2016).
Obesity Connection: Among individuals with obesity, the prevalence of NAFLD increases to 50%–75%, depending on the population studied (Younossi et al., 2018).
Projections: By 2030, the prevalence of NAFLD is expected to increase by 21%–33% globally due to rising rates of obesity, diabetes, and sedentary lifestyles (Estes et al., 2018).
Mechanisms Linking Obesity and NAFLD
Excess visceral fat plays a pivotal role in the development and progression of NAFLD through the following mechanisms:
Increased Free Fatty Acids (FFAs): Adipose tissue in obesity releases excessive FFAs into the bloodstream. These FFAs are transported to the liver, where they accumulate as triglycerides, leading to steatosis.
Insulin Resistance: A hallmark of obesity, insulin resistance impairs the liver’s ability to regulate glucose and lipid metabolism, exacerbating fat deposition.
Inflammation and Oxidative Stress: Obesity promotes chronic low-grade inflammation via pro-inflammatory cytokines like TNF-α and IL-6. These cytokines contribute to liver injury, inflammation, and fibrosis.
Gut Dysbiosis: Alterations in the gut microbiota in obese individuals may promote liver fat accumulation through increased production of short-chain fatty acids and endotoxins.
Potential Complications of NAFLD
NAFLD is not merely a benign condition but a progressive disease with significant complications, including:
Non-Alcoholic Steatohepatitis (NASH):
Approximately 20%–30% of NAFLD cases progress to NASH (Younossi et al., 2018).
NASH is characterized by liver inflammation and damage, increasing the risk of fibrosis.
Liver Fibrosis and Cirrhosis:
Advanced fibrosis is present in 10%–20% of NASH patients. Once cirrhosis develops, patients are at high risk for liver failure and portal hypertension.
Hepatocellular Carcinoma (HCC):
NASH-related cirrhosis is a leading cause of HCC, with NAFLD accounting for 14%–20% of all cases of HCC globally (Younossi et al., 2016).
Cardiovascular Disease (CVD):
Cardiovascular disease is the most common cause of death in patients with NAFLD, driven by systemic inflammation, insulin resistance, and dyslipidemia.
Chronic Kidney Disease (CKD):
Patients with NAFLD have a higher risk of CKD due to shared risk factors, such as hypertension and diabetes.
Type 2 Diabetes Mellitus (T2DM):
NAFLD and T2DM have a bidirectional relationship, with each condition exacerbating the severity of the other.
Risk Ratios for NAFLD and NASH
Obesity is the most significant modifiable risk factor for NAFLD and its progression. Key data include:
Individuals with obesity have a 4.6-fold increased risk (RR: 4.6) of developing NAFLD compared to those of normal weight (Younossi et al., 2016).
For severe obesity (BMI ≥40), the risk increases to 7.2 times (RR: 7.2) (Estes et al., 2018).
Among those with NAFLD, obesity increases the risk of progression to NASH by 1.8 times (RR: 1.8) and to advanced fibrosis by 2.5 times (RR: 2.5) (Younossi et al., 2016).
Conclusion
Non-alcoholic fatty liver disease is a growing global health crisis, driven by the obesity epidemic. While NAFLD begins as a benign fat deposition, it has the potential to progress to life-threatening conditions such as NASH, cirrhosis, and liver cancer. With the increasing prevalence of obesity, the burden of NAFLD is expected to rise sharply, underscoring the urgent need for effective prevention and management strategies. Addressing obesity through lifestyle changes, medical interventions, and public health policies is paramount to curbing the rise of NAFLD and its complications.
5. Obesity and Sleep Apnea
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repeated episodes of partial or complete obstruction of the upper airway during sleep, leading to intermittent breathing pauses, reduced oxygen levels, and disrupted sleep. These breathing interruptions are subtle and not always associated with outright waking up but rather to shallower or poor-quality sleep therefore rendering one’s sleep less restful. OSA is can be accompanied by loud snoring, daytime fatigue, and reduced cognitive function but these symptoms are not all necessary to make the diagnosis. A sleep study on the end is paramount in making the diagnosis. OSA can significantly impact overall health if left untreated, contributing to cardiovascular disease, metabolic dysfunction, and decreased quality of life.
Obesity is widely recognized as the most significant risk factor for OSA, with excess weight directly contributing to airway obstruction and increased severity of the condition. The prevalence of OSA rises dramatically with increasing BMI, particularly in individuals with central (upper body) obesity.
Mechanisms Linking Obesity to Sleep Apnea
The pathophysiological relationship between obesity and OSA is primarily driven by anatomical and metabolic changes caused by excess fat:
Upper Airway Fat Deposition: Excess fat tissue accumulates around the neck, throat, and upper airway. This narrows the air passage and increases the likelihood of its collapse during sleep when muscle tone naturally decreases.
Abdominal Obesity and Reduced Lung Volume: Central obesity, characterized by excess fat around the abdomen and chest, reduces lung volume and limits diaphragmatic movement. This creates negative pressure in the airway, further predisposing individuals to airway collapse.
Inflammation and Hormonal Effects: Obesity is associated with systemic inflammation and altered levels of hormones like leptin, which regulate respiratory drive and muscle tone. Elevated leptin levels in obese individuals may impair the brain's ability to regulate breathing during sleep (Kapsimalis & Kryger, 2009).
These mechanisms explain why obese individuals, particularly those with a BMI ≥30, are significantly more likely to experience OSA and its complications.
Risk Ratios and Prevalence
Obesity greatly increases the risk of developing OSA, with multiple studies demonstrating a strong statistical relationship between BMI and the condition:
Individuals with obesity are at a 6-fold higher risk (RR: 6.0) of developing moderate to severe OSA compared to normal-weight individuals (Peppard et al., 2000).
For every 10% increase in body weight, the risk of OSA increases by approximately 32% (Young et al., 2005).
In severely obese individuals (BMI ≥40), the prevalence of OSA may reach as high as 60%–90% (Kuna & Sant’Ambrogio, 2008).
This association highlights the importance of recognizing and addressing obesity as a primary driver of sleep apnea.
Complications of Untreated Obstructive Sleep Apnea in Obesity
When left untreated, OSA in obese individuals can lead to severe health complications, including:
Cardiovascular Disease: OSA causes intermittent hypoxia (low oxygen levels) and increased sympathetic nervous system activity, which can lead to hypertension, arrhythmias, and increased risk of stroke and heart failure (Somers et al., 2008).
Type 2 Diabetes: OSA and obesity are both associated with insulin resistance. Repeated episodes of hypoxia impair glucose metabolism and exacerbate diabetes risk (Pamidi et al., 2012).
Cognitive Dysfunction: Chronic sleep disruption impairs cognitive function, memory, and focus, significantly reducing daytime productivity and quality of life.
Depression and Anxiety: Poor sleep quality and chronic fatigue in OSA patients often lead to mood disturbances and increased rates of depression.
Reduced Life Expectancy: Severe, untreated OSA has been associated with a 2- to 3-fold increase in mortality risk due to cardiovascular complications and accidents related to daytime sleepiness (Marshall et al., 2008).
Conclusion
Obesity and obstructive sleep apnea share a deeply intertwined relationship, with excess weight serving as a primary cause and exacerbating factor for OSA. The risk of sleep apnea rises dramatically with increasing BMI, and untreated OSA in obese individuals can lead to significant cardiovascular, metabolic, and cognitive complications. Addressing obesity as a primary risk factor for OSA is essential for mitigating its far-reaching health consequences.
6. Obesity and Low Testosterone
Obesity is strongly linked to hypogonadism, a condition characterized by low testosterone levels in men. Testosterone is a critical hormone responsible for regulating male sexual health, fertility, muscle mass, bone density, energy levels, and mood. Low testosterone, commonly referred to as “low T,” can significantly affect quality of life and is increasingly recognized as a consequence of excess body fat.
Mechanisms Linking Obesity and Low Testosterone
The association between obesity and low testosterone is multifactorial, driven by hormonal, inflammatory, and metabolic changes related to excess fat tissue:
Aromatization of Testosterone into Estrogen:
Fat tissue, particularly visceral adipose tissue, contains the enzyme aromatase, which converts testosterone into estradiol, a form of estrogen. In obese men, increased fat tissue leads to higher aromatase activity, resulting in elevated estrogen levels and a subsequent suppression of testosterone production. This creates a negative feedback loop in the hypothalamic-pituitary-gonadal (HPG) axis, further reducing testosterone synthesis (Kelly & Jones, 2013).Inflammatory Effects:
Obesity is associated with chronic low-grade inflammation. Adipose tissue releases pro-inflammatory cytokines such as TNF-alpha and IL-6, which interfere with the signaling pathways involved in testosterone production. These inflammatory molecules impair the function of Leydig cells in the testes, which are responsible for producing testosterone (Gao et al., 2017).Insulin Resistance and Metabolic Dysfunction:
Obesity often leads to insulin resistance and metabolic syndrome, both of which are closely tied to low testosterone. Insulin resistance reduces sex hormone-binding globulin (SHBG) levels, which are necessary to transport testosterone in the bloodstream. Lower SHBG levels contribute to decreased circulating testosterone levels (Corona et al., 2013).Leptin Dysregulation:
In obesity, elevated levels of leptin—a hormone produced by fat cells—can interfere with the normal regulation of the HPG axis. High leptin levels have been shown to inhibit testosterone synthesis in the testes by reducing luteinizing hormone (LH) stimulation of Leydig cells (Tena-Sempere, 2013).
These combined mechanisms create a complex interplay between obesity, hormonal imbalance, and testosterone deficiency.
Clinical Consequences of Low Testosterone in Obese Men
Low testosterone caused by obesity can lead to a range of physical, reproductive, and psychological issues:
Reduced Libido and Sexual Dysfunction: Low testosterone is a major contributor to decreased libido, erectile dysfunction, and impaired sexual performance.
Infertility: Testosterone is essential for spermatogenesis (sperm production). Low testosterone levels can impair fertility by reducing sperm count and motility.
Muscle Weakness and Reduced Lean Mass: Testosterone plays a vital role in maintaining muscle mass and strength. Hypogonadism leads to muscle atrophy, reduced physical performance, and increased frailty.
Increased Fat Mass and Worsening Obesity: Low testosterone can exacerbate obesity, creating a vicious cycle. Testosterone deficiency reduces energy expenditure and promotes fat accumulation, particularly visceral fat, which further lowers testosterone levels.
Bone Loss and Osteoporosis: Chronic low testosterone levels can decrease bone density, increasing the risk of osteoporosis and fractures.
Fatigue, Depression, and Cognitive Decline: Testosterone is involved in regulating mood and cognitive function. Low levels are linked to fatigue, irritability, depression, and impaired memory.
Statistical Evidence: Risk of Low Testosterone in Obesity
The relationship between obesity and testosterone deficiency is well-established in clinical research:
Obese men are 2.4 times more likely (OR: 2.4) to have low testosterone levels compared to men of normal weight (Corona et al., 2013).
The prevalence of low testosterone among obese men (BMI ≥30) is estimated to be as high as 50%, compared to approximately 20% in men with normal weight (Gao et al., 2017).
In severely obese men (BMI ≥40), testosterone levels can be up to 50% lower than those observed in normal-weight individuals (Kelly & Jones, 2013).
These findings underscore the significant impact of obesity on male reproductive and hormonal health.
Conclusion
Obesity and low testosterone are intricately linked through hormonal disruption, inflammation, and metabolic dysfunction. Excess adipose tissue not only lowers testosterone production through estrogen conversion but also perpetuates a cycle of increasing fat accumulation and worsening hypogonadism. The consequences of low testosterone in obese men extend beyond sexual and reproductive health, affecting muscle mass, energy levels, mental health, and overall quality of life. Addressing obesity is therefore a crucial step in restoring healthy testosterone levels and improving long-term health outcomes for men.
7. Obesity and Dementia
Emerging evidence strongly links midlife obesity to an increased risk of cognitive decline and dementia later in life. Dementia is an umbrella term for progressive neurodegenerative conditions that impair memory, cognition, behavior, and functional independence. Alzheimer’s disease (AD) is the most common form, accounting for 60–80% of cases, followed by vascular dementia and other subtypes. Obesity, particularly during midlife, accelerates these processes and significantly increases the risk of dementia and Alzheimer's disease in older age.
Mechanisms Linking Obesity to Dementia
The association between obesity and dementia is multifaceted, with several overlapping pathways contributing to cognitive decline:
Vascular Damage and Cerebral Blood Flow Impairment:
Obesity is a major risk factor for cardiovascular disease, hypertension, and atherosclerosis, all of which impair blood flow to the brain. Reduced cerebral blood flow increases the risk of silent strokes, microvascular damage, and ischemic events, which are key contributors to vascular dementia and Alzheimer’s disease (Whitmer et al., 2005).Chronic Inflammation:
Obesity promotes systemic, low-grade inflammation due to the release of pro-inflammatory cytokines such as IL-6 and TNF-alpha from adipose tissue. These inflammatory molecules can cross the blood-brain barrier, triggering neuroinflammation and oxidative stress, both of which damage neurons and accelerate neurodegeneration (Kivipelto et al., 2005).Metabolic Dysregulation and Insulin Resistance:
Obesity-induced insulin resistance, often a precursor to type 2 diabetes, also plays a central role in cognitive decline. Insulin resistance reduces the brain's ability to utilize glucose, its primary energy source, leading to brain cell dysfunction. Additionally, insulin resistance interferes with the clearance of amyloid-beta peptides, increasing the formation of amyloid plaques, which are hallmark features of Alzheimer’s disease (Benedict & Grillo, 2018).Amyloid Plaque and Tau Protein Accumulation:
Obesity accelerates the development of amyloid plaques and tau protein tangles in the brain. Amyloid-beta plaques are protein fragments that accumulate between neurons, disrupting communication and triggering cell death. Tau protein tangles, on the other hand, occur inside neurons and interfere with essential cell functions, further accelerating neurodegeneration (Hsu & Kuo, 2018).Leptin and Adipokine Dysregulation:
Leptin, a hormone produced by fat cells, is known to regulate appetite and metabolism. However, in obesity, leptin resistance occurs, impairing leptin’s neuroprotective effects in the brain. Additionally, altered levels of adipokines (fat-derived hormones) can disrupt brain signaling pathways, further contributing to cognitive dysfunction (Lieb et al., 2009).
Statistical Evidence: Risk Ratios for Dementia and Obesity
The link between obesity and dementia has been consistently observed in longitudinal cohort studies, particularly for midlife obesity:
Individuals with midlife obesity have a 1.6-fold increased risk (RR: 1.6) of developing dementia later in life (Whitmer et al., 2005).
For Alzheimer’s disease specifically, midlife obesity increases the risk by approximately 1.8 times (RR: 1.8) (Kivipelto et al., 2005).
A meta-analysis of 10 studies showed that each 5-unit increase in BMI during midlife is associated with a 33% higher risk of developing dementia (RR: 1.33) (Anstey et al., 2011).
Importantly, the risk appears strongest when obesity occurs in midlife (ages 40–60). In contrast, some studies suggest that obesity in late life may not carry the same risk or could even appear protective, though this likely reflects reverse causation due to preclinical dementia-related weight loss (Kivipelto et al., 2005).
Potential Complications of Obesity-Related Dementia
Obesity-driven cognitive decline and dementia lead to several severe outcomes:
Progressive Memory Loss: Impaired ability to recall information, affecting daily functioning.
Executive Dysfunction: Difficulty with decision-making, planning, and problem-solving.
Behavioral Changes: Increased incidence of depression, anxiety, apathy, and irritability.
Functional Decline: Loss of independence, requiring long-term care.
Increased Mortality Risk: Individuals with obesity and dementia face higher mortality due to a combination of cardiovascular and neurological complications (Hsu & Kuo, 2018).
Conclusion
Obesity in midlife significantly increases the risk of dementia and cognitive decline later in life through mechanisms including vascular damage, chronic inflammation, insulin resistance, and neurotoxic protein accumulation. Midlife obesity is particularly concerning, with a well-established 1.6- to 1.8-fold higher risk for dementia compared to individuals with normal weight. Addressing obesity during midlife is therefore a critical strategy for reducing the burden of dementia and promoting long-term brain health.
Summary Table 2: Obesity-Associated Risks
Below is a summary table that highlights the relative risk (RR) for each complication associated with obesity.
Summary Chart: Obesity-Associated Risks
Below is a chart that visually highlights the relative risk (RR) for each complication associated with obesity.
Summary:
This chapter addresses the critical, yet often uncomfortable, topic of weight and its significant impact on overall health. Despite its importance, weight remains taboo in clinical medicine, with providers sometimes avoiding direct conversations about obesity due to concerns about stigma, judgment, and time constraints. However, the prevalence of overweight and obesity, which affects 73.6% of American adults, underscores the need for sensitive and proactive discussions around maintaining a healthy weight (Centers for Disease Control and Prevention [CDC], 2022).
The chapter begins by defining weight categories through commonly used tools like Body Mass Index (BMI) and its limitations. While BMI remains widely adopted, more advanced methods such as DEXA scans and waist-to-hip ratios provide a deeper understanding of body composition and associated risks. This nuanced approach allows for better evaluation of weight's effects on health.
The text then explores the health consequences of obesity, emphasizing its role in chronic diseases. Obesity increases the risk of:
Heart Disease: Risk is 2.1-fold higher in obese individuals (Lavie et al., 2009).
Type 2 Diabetes: Obesity raises the risk by up to 7.6-fold for morbidly obese individuals (Hu et al., 2001).
Cancer: Obesity is associated with endometrial, breast, colorectal, and liver cancers, among others, with risk ratios ranging from 1.2 to 6.25 depending on the cancer type and BMI level (Renehan et al., 2008; Bhaskaran et al., 2014).
Sleep Apnea: Obese individuals have a 6-fold higher risk of developing moderate to severe sleep apnea (Peppard et al., 2000).
Dementia: Midlife obesity increases the risk of dementia by 1.6-fold due to mechanisms like vascular damage and chronic inflammation (Whitmer et al., 2005).
The chapter also highlights the lesser-discussed risks of being underweight or undernourished, such as weakened immunity, osteoporosis, and cardiovascular complications. These issues reinforce the importance of achieving and maintaining a healthy weight, not just avoiding obesity.
In conclusion, maintaining a healthy weight through balanced nutrition, regular physical activity, and, when necessary, medical interventions is essential for reducing the risk of chronic diseases, enhancing quality of life, and promoting longevity. This chapter calls for compassionate, evidence-based approaches to weight management, empowering individuals to make informed health decisions.
See obesity newsletter for management and treatment options for obesity.
References
Abnet, C. C., et al. (2018). Obesity and esophageal adenocarcinoma: Pathophysiology and epidemiology. Cancer Epidemiology Biomarkers & Prevention, 27(8), 873–880. https://doi.org/10.1158/1055-9965.EPI-18-0166
Anstey, K. J., Cherbuin, N., & Budge, M. (2011). Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obesity Reviews, 12(5), e426–e437. https://doi.org/10.1111/j.1467-789X.2010.00825.x
Ashwell, M., & Gibson, S. (2016). Waist-to-height ratio as a screening tool for cardiometabolic risk: A systematic review. Obesity Reviews, 17(9), 733–746. https://doi.org/10.1111/obr.12407
Aune, D., et al. (2012). Anthropometric factors and pancreatic cancer risk: A systematic review and dose-response meta-analysis of cohort studies. Annals of Oncology, 23(4), 843–852. https://doi.org/10.1093/annonc/mdr331
Benedict, C., & Grillo, C. A. (2018). Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: A state-of-the-art review. Frontiers in Neuroscience, 12, 215. https://doi.org/10.3389/fnins.2018.00215
Bhaskaran, K., et al. (2014). Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. The Lancet, 384(9945), 755–765. https://doi.org/10.1016/S0140-6736(14)60892-8
Calle, E. E., & Thun, M. J. (2004). Obesity and cancer. Oncogene, 23(38), 6365–6378. https://doi.org/10.1038/sj.onc.1207751
Centers for Disease Control and Prevention. (2020). Defining Adult Overweight and Obesity. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention. (2022). Obesity and overweight. National Center for Health Statistics. Retrieved September 20, 2023, from https://www.cdc.gov/nchs/fastats/obesity-overweight.htm
Chalasani, N., et al. (2018). The diagnosis and management of non-alcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology, 67(1), 328–357. https://doi.org/10.1002/hep.29367
Corona, G., Monami, M., Rastrelli, G., Aversa, A., Sforza, A., Lenzi, A., & Maggi, M. (2013). Testosterone and metabolic syndrome: A meta-analysis study. The Journal of Sexual Medicine, 10(10), 2477–2485. https://doi.org/10.1111/jsm.12255
Estes, C., et al. (2018). Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States. Journal of Hepatology, 69(4), 896–904. https://doi.org/10.1016/j.jhep.2018.05.036
Gao, Q., Zhao, X., Zhang, Z., & Zhang, Z. (2017). The relationship between testosterone levels and obesity in men: A meta-analysis. Asian Journal of Andrology, 19(6), 643–650. https://doi.org/10.4103/aja.aja_31_17
Grant, R. L. (2014). Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ, 348, f7450. https://doi.org/10.1136/bmj.f7450
Heymsfield, S. B., & Wadden, T. A. (2017). Mechanisms, pathophysiology, and management of obesity. New England Journal of Medicine, 376(3), 254–266. https://doi.org/10.1056/NEJMra1514009
Hsu, T. M., & Kuo, Y. M. (2018). The interplay between obesity and Alzheimer’s disease. Neurobiology of Disease, 118, 33–42. https://doi.org/10.1016/j.nbd.2018.07.013
Hu, F. B., et al. (2001). Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New England Journal of Medicine, 345(11), 790–797. https://doi.org/10.1056/NEJMoa010492
Kapsimalis, F., & Kryger, M. H. (2009). Sleep breathing disorders in the U.S. female population. Journal of Women's Health, 18(8), 1211–1219. https://doi.org/10.1089/jwh.2008.1170
Kivipelto, M., Ngandu, T., Fratiglioni, L., Viitanen, M., Kåreholt, I., Winblad, B., ... & Nissinen, A. (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology, 62(10), 1556–1560. https://doi.org/10.1001/archneur.62.10.1556
Kelly, D. M., & Jones, T. H. (2013). Testosterone and obesity. Obesity Reviews, 16(7), 581–606. https://doi.org/10.1111/obr.12282
Kuna, S. T., & Sant’Ambrogio, G. (2008). Pathophysiology of upper airway closure during sleep. Journal of Applied Physiology, 64(1), 403–410.
Lauby-Secretan, B., et al. (2016). Body fatness and cancer: Viewpoints of the IARC Working Group. New England Journal of Medicine, 375(8), 794–798. https://doi.org/10.1056/NEJMsr1606602
Lavie, C. J., et al. (2009). Obesity and cardiovascular disease risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology, 53(21), 1925–1932. https://doi.org/10.1016/j.jacc.2008.12.068
Lieb, W., Beiser, A. S., Vasan, R. S., Tan, Z. S., Au, R., Harris, T. B., ... & Seshadri, S. (2009). Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA, 302(23), 2565–2572. https://doi.org/10.1001/jama.2009.1836
Marshall, N. S., Wong, K. K., Liu, P. Y., Cullen, S. R., Knuiman, M. W., & Grunstein, R. R. (2008). Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep, 31(8), 1079–1085. https://doi.org/10.1093/sleep/31.8.1079
Norton, E. C., Dowd, B. E., & Maciejewski, M. L. (2018). Odds ratios—current best practice and use. JAMA, 320(1), 84–85. https://doi.org/10.1001/jama.2018.6971
Pamidi, S., Wroblewski, K., Stepien, M., Sharif, S., & Tasali, E. (2012). Obstructive sleep apnea in young lean men: Impact on insulin sensitivity and secretion. Diabetes Care, 35(11), 2384–2389. https://doi.org/10.2337/dc12-0842
Peppard, P. E., Young, T., Palta, M., Dempsey, J., & Skatrud, J. (2000). Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA, 284(23), 3015–3021. https://doi.org/10.1001/jama.284.23.3015
Renehan, A. G., et al. (2008). Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. The Lancet, 371(9612), 569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Somers, V. K., White, D. P., Amin, R., Abraham, W. T., Costa, F., Culebras, A., ... & Young, T. (2008). Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation scientific statement. Circulation, 118(10), 1080–1111. https://doi.org/10.1161/CIRCULATIONAHA.107.189375
Tena-Sempere, M. (2013). Interaction between energy homeostasis and reproduction: Central effects of leptin and ghrelin on the reproductive axis. Hormone and Metabolic Research, 45(11), 919–927. https://doi.org/10.1055/s-0033-1357172
Whitmer, R. A., et al. (2005). Obesity in middle age and future risk of dementia: A 27-year longitudinal study. BMJ, 330(7504), 1360. https://doi.org/10.1136/bmj.38446.466238.E0
World Cancer Research Fund. (2021). Obesity and cancer risk. Retrieved from https://www.wcrf.org
Young, T., Finn, L., Peppard, P. E., Szklo-Coxe, M., Austin, D., Nieto, F. J., ... & Hla, K. M. (2005). Sleep-disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep, 31(8), 1071–1078.
Younossi, Z. M., et al. (2016). Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology, 64(1), 73–84. https://doi.org/10.1002/hep.28431
Younossi, Z. M., et al. (2018). Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology, 68(5), 1537–1545. https://doi.org/10.1002/hep.29824